Forward Osmosis (FO)
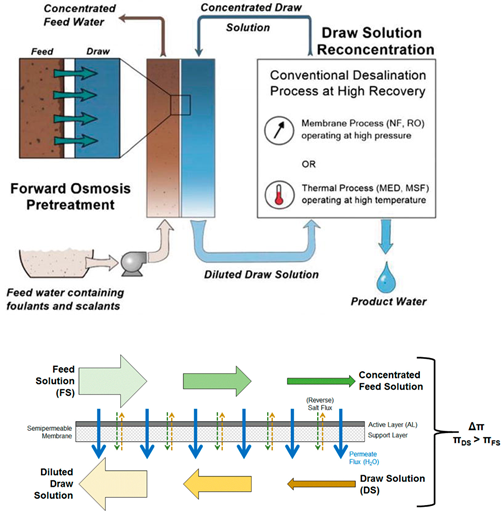
Forward osmosis (FO) is a membrane separation process. The principle of an FO process relies on using the natural osmotic process to draw the water molecules across a semi- permeable membrane from a lower concentrated solution or lower chemical potential (feed) to a higher concentrated solution or higher chemical potential, namely the draw solution (DS). FO has applications in separation processes for wastewater treatment, food processing, and seawater/brackish water desalination and also concurrent treatment of saline water and wastewater.
Membrane Distillation (MD)
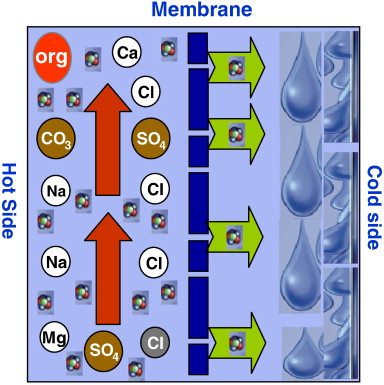
MD is an emerging non isothermal membrane process for separation mainly suited for applications in which water is the major component present in the feed stream to be treated. This membrane process refers to a thermal driven transport of vapor through a microporous hydrophobic membrane, the driving force being the partial pressure difference between each side of the membrane process. In other words, MD is based on the phenomenon in which pure water in the vapor state can be extracted from aqueous solutions, with the vapor passing through the membranes pores. The temperature difference leads to a vapor pressure difference across the membrane. Due to the hydrophobic nature of the membrane, only vapor can pass through the membrane and not the liquid solution being distilled.
Hemodialysis
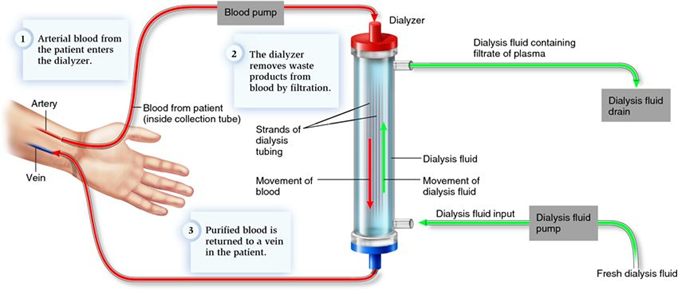
Hemodialysis is a process of purifying the blood of a person whose kidneys are not working normally. In the other word, hemodialysis is a treatment process that directly removes the pathogenic substance or toxic substance from the patients’ blood by means of extracorporeal circulation equipment. To achieve this purpose, thousands of porous hollow fiber membranes are fitted into a single membrane module commonly known as hemodialyzer. In general, the heart of the hemodialysis machine is the hemodialyzer, where semipermeable membranes are arranged in the middle, serves as membrane contactor to form separate adjacent paths for blood and dialysate.
Membrane Bioreactors (MBR)
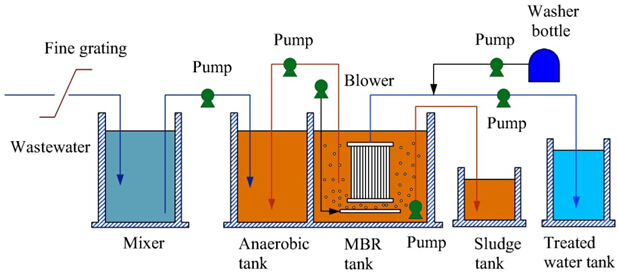
The combination of biological processes with membrane separation is one of the newest rapidly growing sewage treatment technologies in the industry known as Membrane bioreactor (MBR). Low-pressure membranes (such as microfiltration or ultrafiltration) are commonly used in MBRs. The common filtration systems have a sewage aeration unit, secondary clarifier and possibly third-order filters. When wastewater treatment uses aerobic or anaerobic biological processes, system stability and refined water quality are usually dependent on the remaining sludge separation at the end of the process. Today, membrane bioreactors are used to treat various types of wastewater, such as municipal sewage, high-organic wastewater and heavy wastewater. There are two types of membrane systems that can be used in MBRs: First, cartridges inside the tubing with vacuum propulsion, which are designed to be installed inside the membrane bioreactor. Second, submerged membrane technologies use hollow fiber membranes or flat plates. Due to the need for low pressure (vacuum force) to be easier to operate and adapt to changes in the types of solids contained in active sludge bioreactors and lower final cost, especially in utility installations, the most common uses are membrane bioreactors.
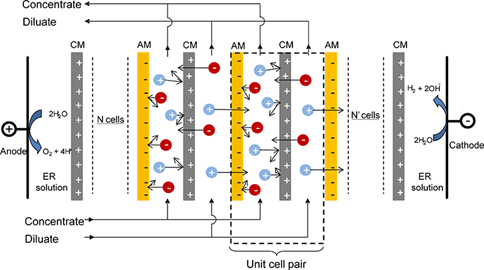
Electrodialysis (ED)
Electrodialysis (ED) is a type of technology that arranges ion-exchange membranes alternately in a direct current field. As it is known there are at least five major elements complementary for ED applications: (1) direct current supply, which proves effective to reinforce ion migration; (2) electrodes, where the oxidation/reduction reactions occur to realize the transformation from ionic conduction to electron conduction and thus provide the original driving force for ion migration; (3) ion exchange membranes, the key components which permit the transport of counter ions and block the passage of co-ions; (4) solvents, which make a media for ion transport by filling the space between the electrodes and membranes; (5) electrolyte, the current i.e. electron carrier between cathode and anode.
Gas Separation (GS)
A gas separation membrane preferentially removes one or more components of a gas mixture that is passed across a membrane surface. This process commonly takes place in 3 steps. The gas molecules first dissolve in the membrane, then diffuse across the membrane, and finally desorb from the downstream side with pressure difference acting as driving force. Gas separation operations include for example, recovery of hydrogen, isolating oxygen, capturing of carbon dioxide, gas dehydration etc. The membranes used in gas separation processes are widely made of two types of materials: polymers and inorganic materials such as ceramics and metals. In our research group, we are dealing not only with the novel membrane materials and techniques but also insight understanding of permeation mechanisms.
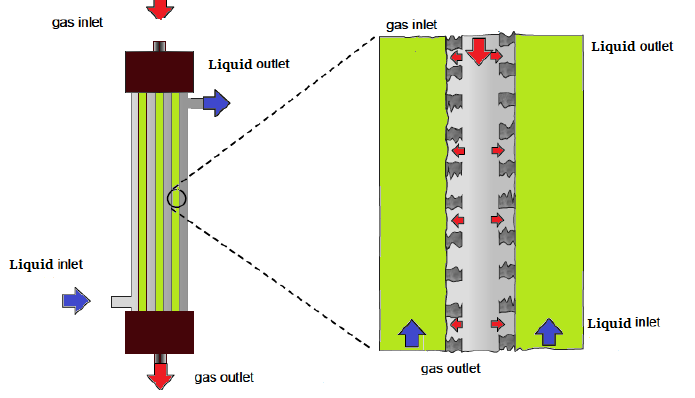
Membrane Contactor (MC)
In a Membrane Contactor, membrane creates a physical barrier between the two streams. Membrane Contactors can be used in several industrial fields such as liquid/liquid extraction, gas absorption and stripping, air dehumidification and humidification, membrane distillation, air conditioning etc. There are two membrane configuration such as hollow fiber and flat sheet for application of this process in gas-liquid contact. By using a suitable membrane configuration, fluids can contact on the opposite sides of the membrane and the fluid-fluid interface is formed at the mouth of each membrane pore. Mass transfer occurs by diffusion across the membrane unlike the traditional contacting equipment. Furthermore, the driving force for separation is concentration difference rather than pressure gradient; indeed for the case of gas-liquid contact only a very small pressure drop across the membrane is required to ensure that the gas-liquid interface remains immobilized at the mouth of the pore. The most important challenges are: pore wetting and membrane material long-term stability during the harsh conditions.
Ceramic membrane fabrication
Ceramic membranes have been known for years and used in many different applications depending on their numerous advantages: stability at high temperatures and pressure resistance, good chemical stability, high mechanical resistance, long life and good antifouling properties. Ceramic membranes can be made from alumina, cordierite, silica, spinel, zirconia, titanium dioxide, silicon carbide, zirconium dioxide, kaolin, pozzolan, quartz, natural zeolite and other refractory oxides. To further improve the separation performance, as an innovative type of ceramic membranes, multilayer ceramic membranes (support and membrane layer) have recently come into sharp focus of both academic and industrial researchers due to their superior proprieties. The supports can typically be shaped by extrusion or isotactic pressing techniques while the membrane layer can generally be prepared by dip-coating, spin-spraying and cross-flow filtration methods. Ceramic membrane can be utilizes in various processes such as water and wastewater treatment, gas separation, membrane distillation, membrane contactor and etc.